Washington D.C.--An upsurge in the public interest has seen cannabidiol (CBD) incorporated into numerous unregulated products. Recently, the FDA underlined the need for a fresh regulatory approach balancing public demand with required oversight to manage risks. This is important given the touted antimicrobial properties of CBD, along with the surge in cannabinoid-infused products aimed at women's care.
[Image via Cannabis Industry Journal]
According to a Cannabis Industry Journal report, only lubricants and tampons fall under FDA regulation, with all other personal care products treated as cosmetics, even those not derived from cannabis. Therefore, they aren't mandated to be tested for their impact on reproductive microbiota. This led us to explore the effects of exogenous cannabis products on key lactobacilli species linked to a healthy reproductive tract. We utilized detailed exposure studies to examine CBD and cannabigerol (CBG)'s antimicrobial activity against L. crispatus, a significant vaginal lactobacilli species.
The complexity of cannabis extract chemoprofiles, driven by cultivars, extraction techniques, and chemical additives, necessitates the adoption of assays that provide vital toxicity data in an in vitro system. The report noted that a recent Israeli study demonstrated inherent immune response biodiversity in cannabis extracts, suggesting the choice of cannabis source should be function-based.
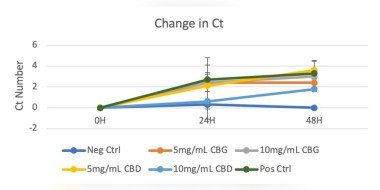
The report noted that the study found that 99% pure CBD and CBG isolates were not inhibitory at the two doses tested by qPCR, turbidity, and plating. Limitations prevent definitive conclusions on which cannabinoids or other secondary cannabis metabolites are inhibitory in vivo to dominant lactobacilli species. Also, the low solubility of CBD in water and its propensity to adhere to unrelated proteins may limit its antimicrobial potential.
Health Canada and the U.S. FDA have concluded the need for appropriate guidelines to manage the risk of CBD products, as per the report. Despite the limitations, the prospect of more research in this field holds promise, potentially benefiting patient outcomes. More basic physiological research is essential to understand the impact of self-administration of CBD preparations based on the exposure route.
Learn more in the Cannabis Industry Journal report.